 |
|||
|
|
|||
|
Page Title:
Biokinetic Model for Soluble OBT |
|
||
| ||||||||||
|
|  DOE-HDBK-1184-2004
calculate ACVo. Accordingly, the most conservative ACVo for ITPs (based on the
CEDE DCFo determined in Section 5.2.4.1 for ITPs [4.3 x 10-10 Sv/Bqo]) is 4.8 104
Bqo/m3 (1.2 Cio/m3, 1.3 10-6 Cio/cm3).
5.2.5
Biokinetic Model for Soluble OBT
For soluble forms of OBT, particularly solvents and the small molecule component
of OBT oil, the assumption can be made that all activity deposited is
instantaneously absorbed into the body (ICRP 78, 1997). Soluble OBT is,
therefore, amenable to available urine bioassay. However, the biokinetic model for
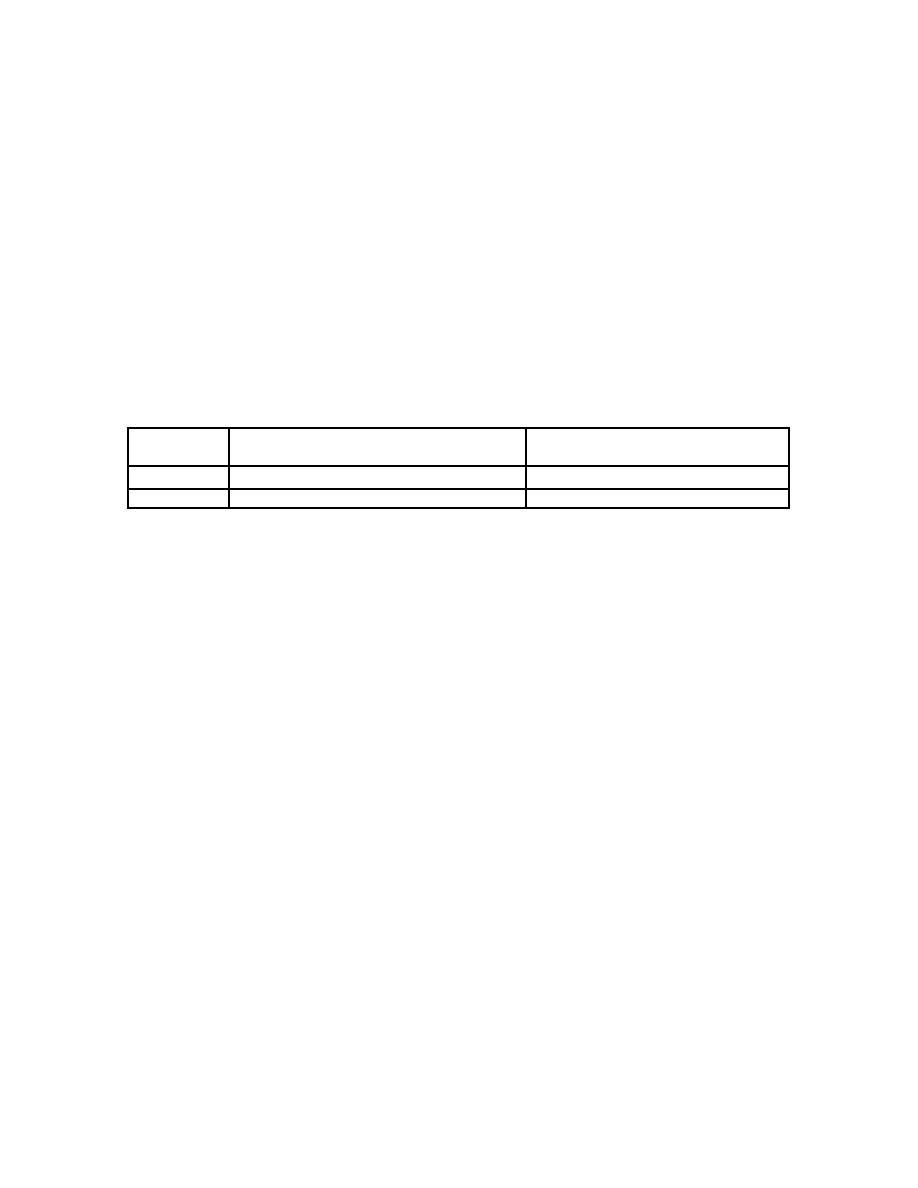
soluble OBT is different from that for HTO. Table 5-15 shows the difference in
fractional uptake and biological halftime for soluble OBTs and HTO (ICRP 78,
1997).
Table 5-15. Fractional Uptake and Biological Halftime for Soluble OBT and HTO (ICRP 78, 1997)
Fractional uptake with 10 day
Fractional uptake with 40 day
biological T1/2
biological T1/2
HTO
97%
3%
OBT
50%
50%
5.2.5.1 Intake and Dose Assessment for Soluble OBT
Soluble OBT solvents or oil components do not cause self-absorption of tritium beta
activity as do solid STCs. A DCF and ACV can therefore be expressed in terms of
actual activity. The CEDE DCF that should be used for a soluble OBT intake is
4.1 10-11 Sv/Bq (152 rem/Ci) (ICRP 78), unless a more appropriate DCF is
derived and documented for the type of soluble OBT encountered in the workplace.
To calculate the ACV for vapors of soluble OBT, there are two pathways that must
be considered (which are similar to HTO pathways), inhalation and skin absorption.
If there is no evidence that a soluble OBT is absorbed through the skin, then uptake
via skin absorption is zero and the ACV can be calculated using Eq 5-12. Using
the DCF for OBTs from ICRP 78 of 4.1 10-11 Sv/Bq, an ACV of 5.0 x 105 Bq/m3 is
calculated.
If there is evidence that an OBT is absorbed via the skin the following approach
should be used to calculate the ACV. ICRP 30 states that for exposure to airborne
HTO, two-thirds (2/3) of the intake is from inhalation and one-third (1/3) is from skin
absorption. Therefore, the total intake of airborne HTO is 1.5 times the inhaled
intake. To determine an ACV, the ICRP 30 assumptions for uptake of airborne
HTO are assumed here to be valid for soluble OBT, i.e., 2/3 of the intake is due to
inhalation and 1/3 is due to skin absorption. Therefore, equation 5-12 should be
divided by a factor of 1.5 to account for the OBT taken into the body from skin
absorption. Accordingly, dividing the result of the calculation from the previous
paragraph by 1.5, the calculated ACV for soluble OBT, that accounts for skin
absorption, is 3.3 105 Bq/m3 (9.1 Ci/m3 or 9.1 10-6 Ci/cm3).
46
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |