 |
|||
|
|
|||
|
Page Title:
Self-Absorption Factor (SAF) for ITPs |
|
||
| ||||||||||
|
|  DOE-HDBK-1184-2004
5.2.3
Self-Absorption Factor (SAF) for ITPs
Within the ITP, the mass of the particulate absorbs a portion of the beta particles
emitted during tritium decay. The fraction of beta particles that escapes the ITP is
called the "self-absorption factor for beta particles" (SAFβ). When analyzing air
monitoring samples for ITPs using LSC, it is necessary to consider SAFβ, since
ITPs do not dissolve appreciably and release tritium to LSC cocktail. Only beta
radiation that escapes the particles in LSC cocktail is available for detection. The
SAFβ is used to estimate the "actual" ITP activity that would be "observed" when
samples are counted via LSC. This use of SAFβ has been confirmed experimentally
(Kropf, 1998), although the supporting evidence is limited.
To assess dose to the lung from ITPs properly, the "self-absorption factor for
energy" (SAFe) is an important consideration. Absorbed dose is a measure of the
energy deposited (per unit mass) in tissue. Only the beta energy that escapes the
particles contributes to dose. SAFe is the fraction of energy that escapes the
particles. Because LuDEP does not account for SAFe, refinement of the results of
LuDEP computations is necessary.
The SAFβ and SAFe for given physical particle sizes are calculated numerically here
by the methods described by Kropf (1998). The determinations of SAFβ and SAFe
are dependent on the electron density of a given material, which is proportional to
A/( Z∆), where A is the atomic mass of the material's empirical formula, Z is the
number of protons in the empirical formula, and ∆ is the density.
The values employed to calculate the self-absorption factors (SAFs) for several
representative materials are given in Table 5-8 below. DOE has handled tritides of
several metals, considered to be insoluble, which do not appear in the table (in the
interest of brevity), including scandium, yttrium, and alloys of titanium and
lanthanum. The derivation of SAFs for a representative but broad range of tritiated
particulate materials is the prime focus of this section. The materials investigated
were selected to represent a wide range of self-absorption, itself a function of the
parameter A/( Z∆). The materials in the tables of this section have values of this
parameter which vary from 0.2 to 1.9. Parameter values for scandium, yttrium, and
lanthanum ditritides are 0.72, 0.52, and 0.47, respectively. [Values for lanthanum
alloys are slightly larger than those for lanthanum because of the inclusion of lighter
elements. Values for iron/titanium alloys are close to those for titanium as given in
the tables.] With respect to SAFs, the materials included in the tables therefore
bracket the metals and alloys not included.
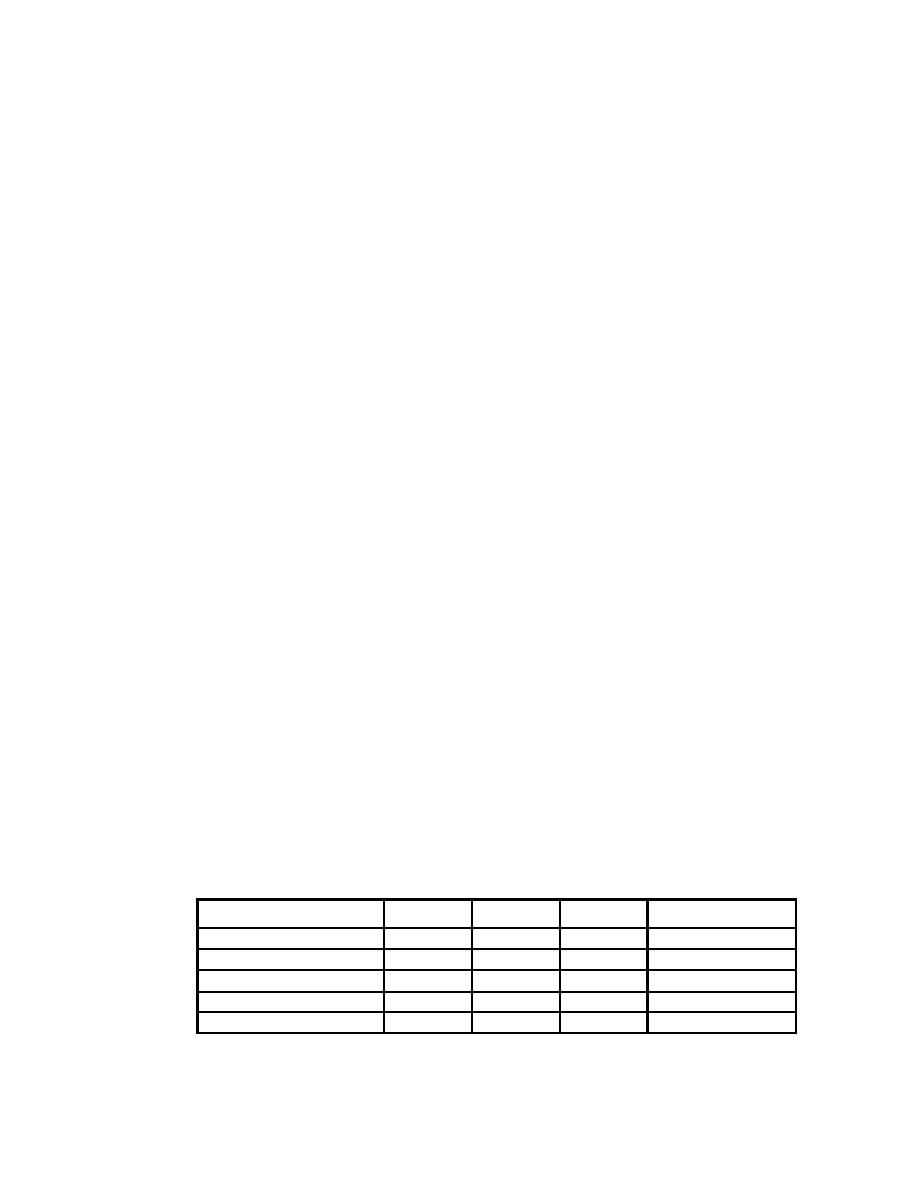
Table 5-8. Constants for calculating SAFs for various monodisperse (s g = 1) ITPs
∆
A/( Z∆)
Base Material*
A
Z
Organic [∼(CH2)n]
14
8
0.9
1.944
Rust [∼FeO(OH)]
89
43
3
0.690
Ti H2
47.9
22
3.9
0.558
Zr H2
91.22
40
6.49
0.351
Hf H2
178.49
72
11.68
0.212
* Variable amounts of elemental hydrogen are isotopically tritium.
39
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |