 |
|||
|
|
|||
|
Page Title:
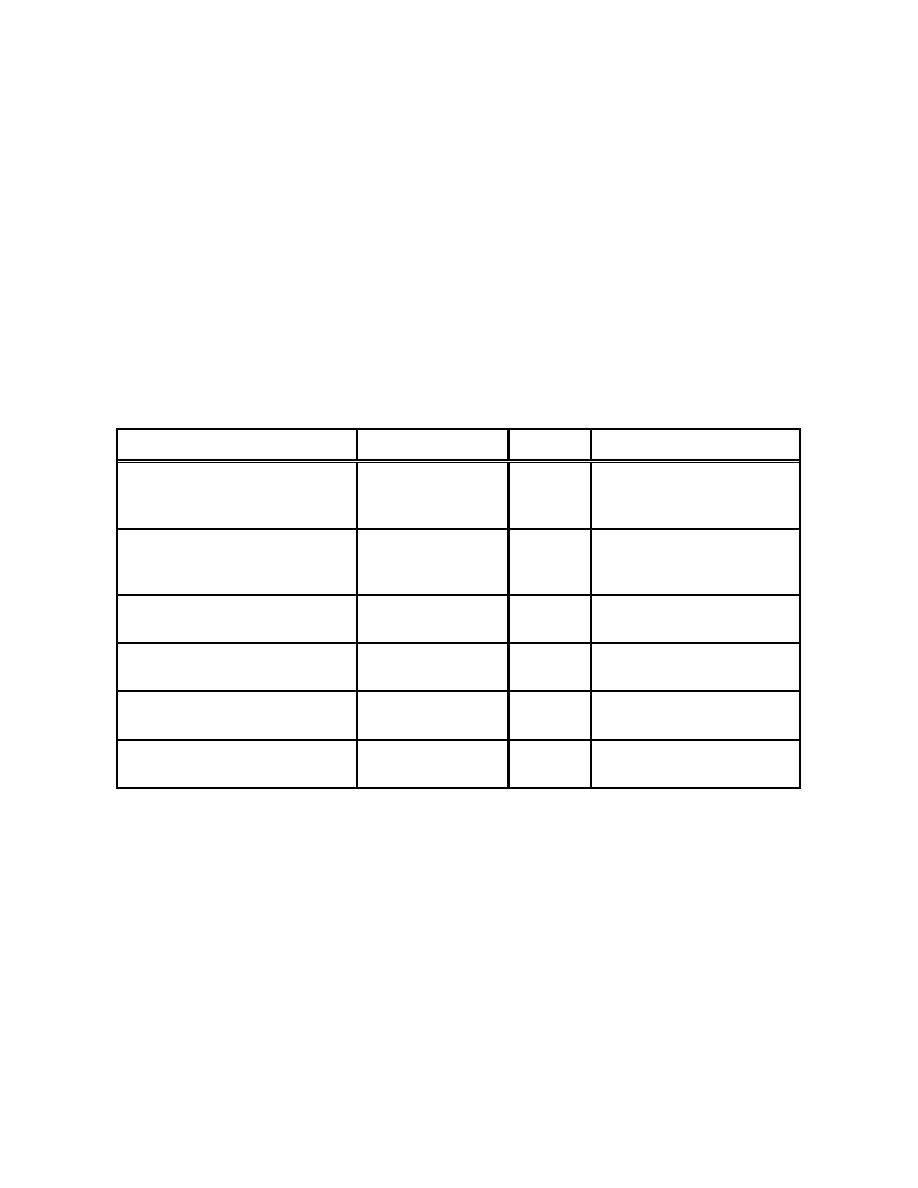
Table 3-13. Measured Uranium ARFs During the Burning of TBP-Kerosine Over Aqueous Phase |
|
||
| ||||||||||
|
|  DOE-HDBK-3010-94
3.0 Liquids; Organic, Combustible Liquids
Figure A.10 of Appendix A. The liquids were heated by heating tapes wrapped around the
metal beaker except in the single case where a borosilicate glass beaker was used. In this
case, radiant heat panels were used. The organic liquid ignited and air (27.5-cfm) was
drawn up the 25.4-cm diameter chimney. Airborne particles were collected on glass fiber
filters as a function of time. The pertinent data taken from Table A.6 in the reference
document (original data tables reproduces as Table A.19 in Appendix A) are tabulated in
Table 3-13.
Table 3-13. M easured Uranium ARFs During the Burning of
TBP-Kerosine Over Aqueous Phase
(Table A.6 - Halverson, Ballinger and Dennis, February 1987)
Solutions Tested
Burn Duration, m in
ARF
Rem ark s
100 ml 30% TBP-kerosine (U) +
27.5
4.04E-3
Aqueous boiled over and
100 ml acid
34.8
5.57E-3
quenched fire, 40% to 60%
53.3
4.34E-3
organic unburned
100 ml 30% TBP-kerosine (U) +
24.8
2.52E-2
Aqueous boiled over and
100 ml acid (FP)
34.0
2.70E-2
quenched fire, 40% to 60%
organic unburned
100 ml 40% TBP-kerosine + 100
61.3
5.98E-2
Burned to dry residue
ml acid (U + FP)
65.0
7.09E-2
Burned to dry residue
50 ml 40% TBP-kerosine + 150 ml
40.0
1.7E-3
~40% organic unburned
acid (U + FP)
50 ml 30% TBP-kerosine (U) +
57.3
1.56E-2
Unburned organic residue
150 ml acid (U + FP)
50 ml 30% TBP-kerosine (U) +
51.0
8.09E-3
Unburned organic residue
100 ml acid (U + FP)
The measured values for ARF appear to have two orders of magnitude variation. The
conservative upper bound ARF is 1E-1. The RF was only measured for one experiment
[50 ml 30% TBP-kerosine (U) + 150 ml acid (U + FP)] with a value of 0.99. In most cases,
heat transferred through the metal solution holder resulted in a boilover that terminated the
burning. Use of glass holders or no external heat addition after ignition delayed boilover.
Only in experiments #52 and #53 (40% TBP in normal paraffin hydrocarbon) using heating
tape to heat the liquid did the burning proceed to complete dryness. It appears that burning
the liquids to dryness increases the ARF; the two highest measured ARFs are from this
configuration (6.0E-2 and 7.1E-2). The variation found for the other experimental
configurations may be partially due to the vigor in boiloff and composition of the aqueous
phase. The presence of salts in the aqueous phase may result in a slightly greater heat
Page 3-45
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |