 |
|||
|
|
|||
|
Page Title:
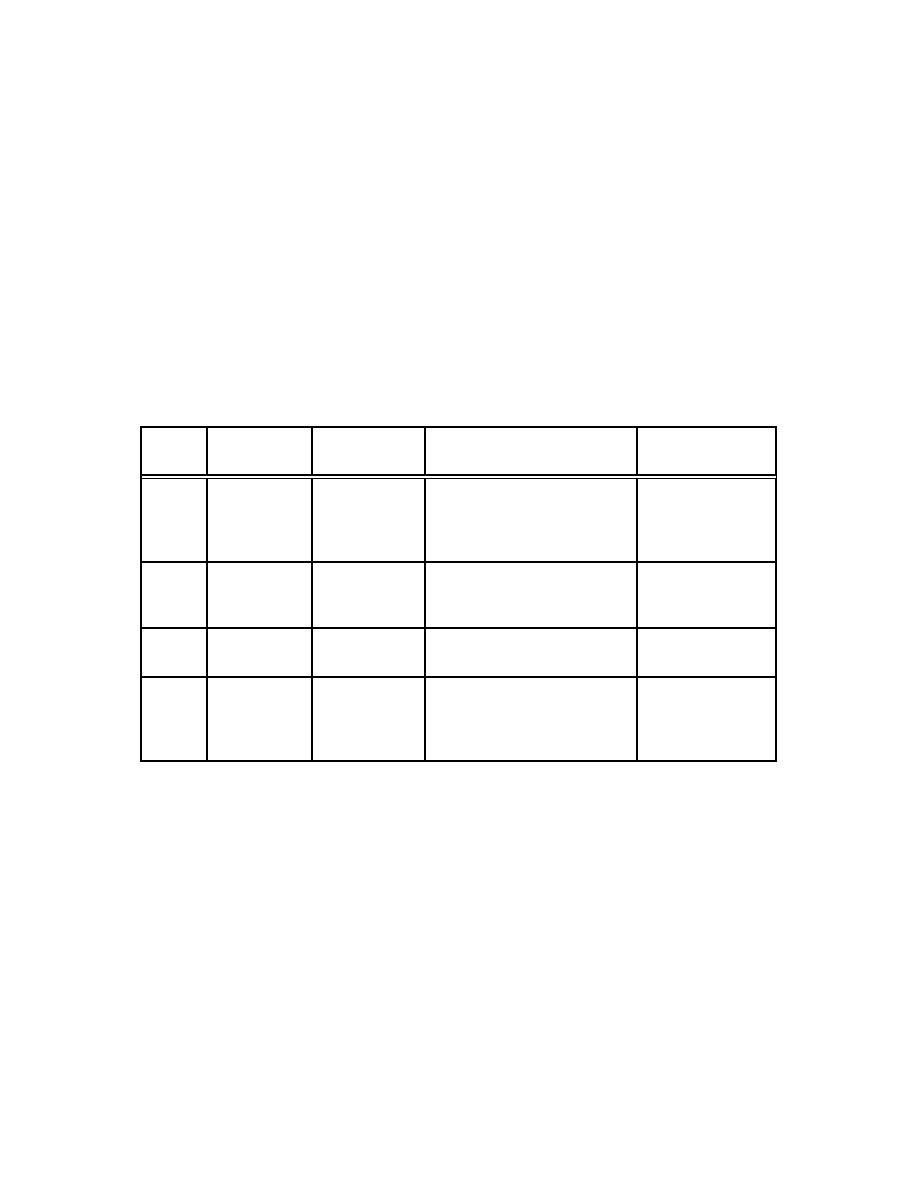
Table 4-2. Sum m ary of Oxidation Rates for Plutonium at Tem peratures Below 100 oC1 |
|
||
| ||||||||||
|
|  DOE-HDBK-3010-94
4.0 Solids; Metals
Equivalent Sphere for oxidation at this temperature is shown as slope B in Figure 4-3 for the
delta-phase and the mass fraction for the RF can be approximated by using the mass fraction
for particles 3 m (i.e., 10 m AED). Under this assumption, an RF between 0.02 and 0.04 is
indicated. If the metal is not completely oxidized in the event, the ARF and RF must be
applied to the fraction of material oxidized. Some indication of the oxidation rate can be
obtained from measured rates (as mg of PuO2/cm2-hr formed) shown in Table 4-2 from early
data taken from the referenced source. Haschke (July 1992) reported "the observed
(oxidation) rate of 0.2 g PuO2/cm2-min is independent of temperature."
Table 4-2. Sum m ary of Oxidation Rates for Plutonium at
Tem peratures Below 100 oC1
(Stewart 1963)
M etal
Tem perature
Relative
Oxidation Rate m g
Source
o
PuO2/cm 2hr
C
Phase
Hum idity %
α
30
95
0.01
Sackman 1960
90
55
15 during 10-60 hr period
200 after initially slower rate2
90
95
100
0
0.04
α
35
20
0.01
Waber et al. 1960
75
0
6E-4
75
50
0.4
α
0.0253
50
0
Dempsey and Kay
50
100
1958
0.04
γ
30
95
0.01
Sackman 1960
90
55
0.2
90
95
0.4
100
0
0.006
1
Data extracted from graphs by the authors quoted and are intended to give a general trend only.
2
The oxidation rates during the initial phase were much smaller; about 2 and 4 mg PuO2/cm2hr at 55 and
95 per cent relative humidity, respectively. More recent studies indicate that onset of such rapid
corrosion is exceptional and may be due to impurities in the metal and its pretreatment (Sackman,
private communication).
3
This rate applied up to 20 hr and thereafter the weight of the sample remained constant.
The airborne release rates of respirable particles can also be estimated from Figure 4-4 and
4-5 reproduced from Chatfield (1968) by converting the rates expressed in activity/cm2-hr to
mass/cm2-hr as shown subsection 4.2.1.1.1. An ARF of 3E-5 is considered to bound the
experimentally measured ARFs at elevated temperatures less than ignition. This value also
bounds most of the data for measured release during the self-sustained oxidation of plutonium
Page 4-20
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |