 |
|||
|
|
|||
|
Page Title:
Figure 3 Neutral and ionized atoms |
|
||
| ||||||||||
|
|  RADIOLOGICAL FUNDAMENTALS
DOE-HDBK-1079-94
Tritium Primer
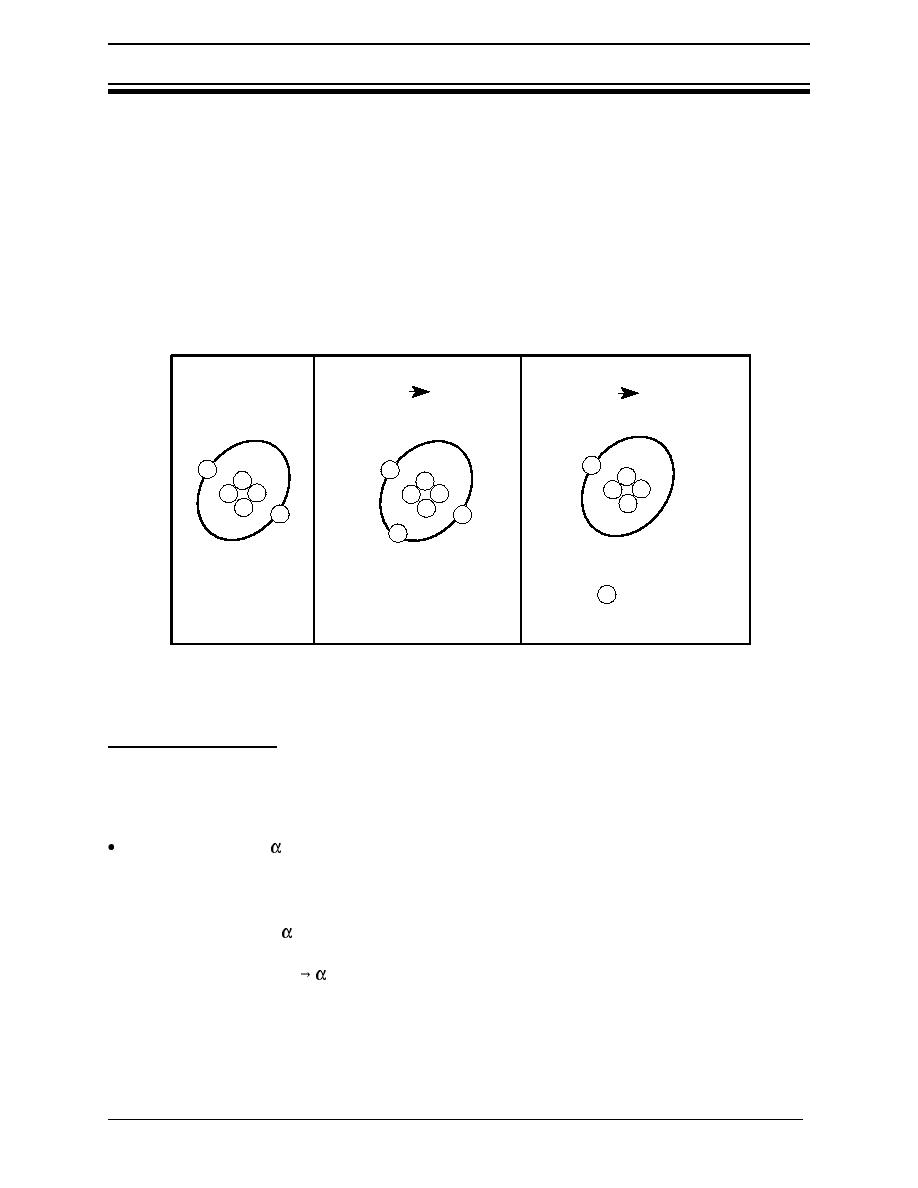
Electrically charged atoms or molecules are called ions. Ions are either positively or negatively
charged, depending on the number of orbiting electrons relative to the number of protons in the
nucleus. As shown in Figure 3, ions with more electrons than protons are negatively charged,
while ions with more protons than electrons are positively charged. The process of breaking a
neutral atom or molecule into electrically charged parts is called ionization. This process
requires energy. Ionization removes electrons from the atom, or molecule, leaving an ion with
a positive charge. The negatively charged electron (which can attach itself to a neutral atom or
molecule) and the positively charged ion, are called an ion pair. Radiation that causes
ionization is called ionizing radiation.
Net
Net
negative
Deficient
positive
Neutral
Excess
charge
electrons
charge
atom
electrons
-
-
-
n
n
n
+
+
+
n
n
n
+
+
+
-
-
-
Captured
electron
Free
-
electron
Figure 3 Neutral and ionized atoms
Types of Radiation
There are four basic types of ionizing radiation emitted from nuclei: alpha particles, beta
particles, gamma rays, and neutrons.
nucleus of a helium atom (4He) (Figure 4). Generally, only the heavy nuclides can emit
alpha particles.
A typical example of an -emitting nuclide is uranium-238:
238
+ 234 Th + energy .
U
92
90
The mass of an alpha particle is about four times the mass of a single neutron or proton, and
has a positive charge of +2 (it has no electrons). This positive charge causes the alpha particle
Tritium
Page 6
Rev. 0
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |