 |
|||
|
|
|||
|
|
|||
| ||||||||||
|
|  Tritium Primer
DOE-HDBK-1079-94
RADIOLOGICAL FUNDAMENTALS
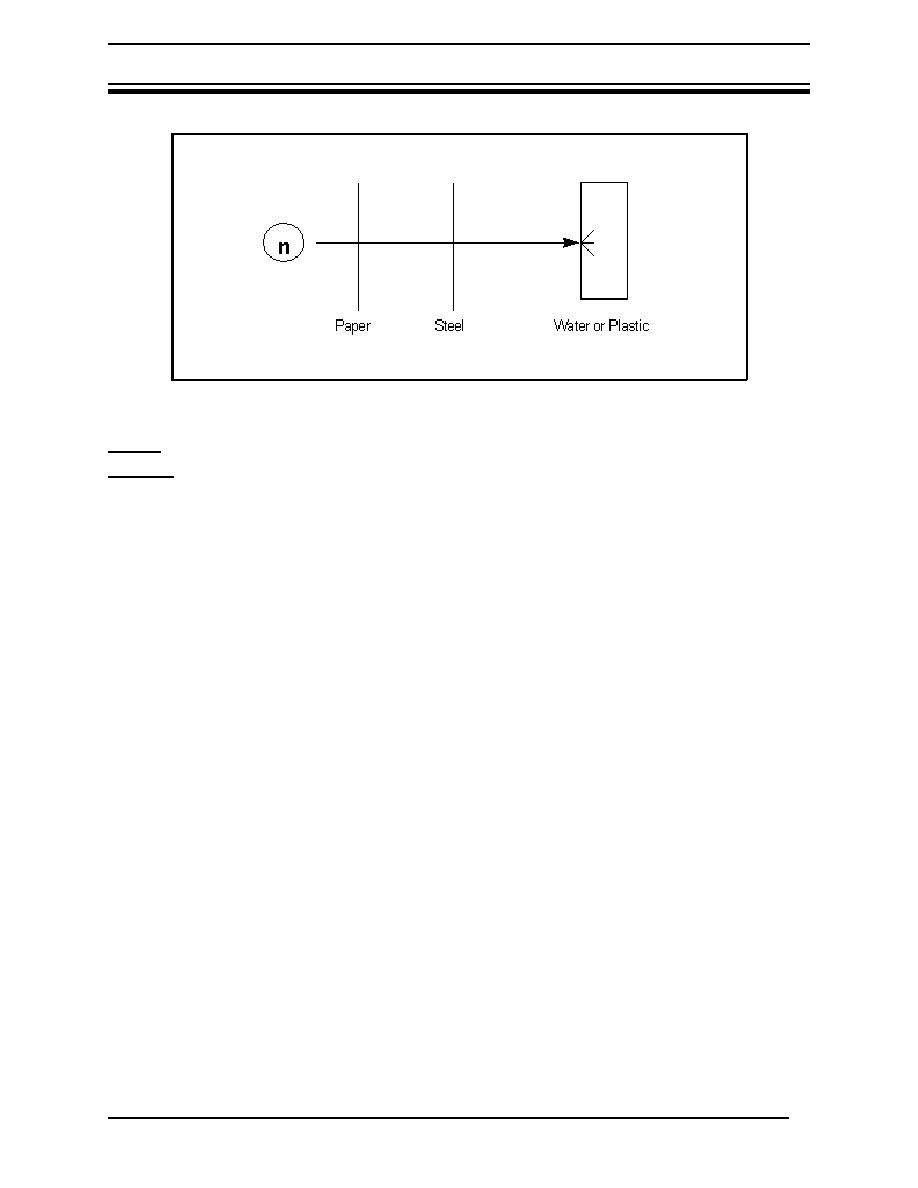
Figure 6 Neutron Shielding
Radio
activity
As radioactive isotopes decay, the number of radioactive nuclei decreases. The time required
for half of the nuclei in a sample of a specific radioactive isotope to undergo decay is called its
(physical) half-life (Figure 7). Each radioactive isotope has its own characteristic half-life.
Radioactive isotopes decay to less than 1% of their original quantity after about seven half-lives.
Half-lives vary widely with different radionuclides, as shown by the following examples:
16
N
-
7.35 seconds
3
H
-
12.43 years
238
4.5 109 years (4.5 billion years)
U
-
The activity of a radioactive isotope sample is defined as the number of nuclei that decay per
unit of time.
It has been shown that for a pure radioactive isotope the number of nuclei decaying per unit time
(rate of decay) is proportional to the number of nuclei available to decay. If the substance is
not being replenished, its activity will decrease accordingly. Therefore, in terms of half-life, the
remaining activity after a period of time can be expressed as follows:
At = Ao (1/2)n
Ao/At = 2n
or
Rev. 0
Page 9
Tritium
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |