 |
|||
|
|
|||
|
|
|||
| ||||||||||
|
|  DOE-HDBK-3010-94
3.0 Liquids; Organic, Combustible Liquids
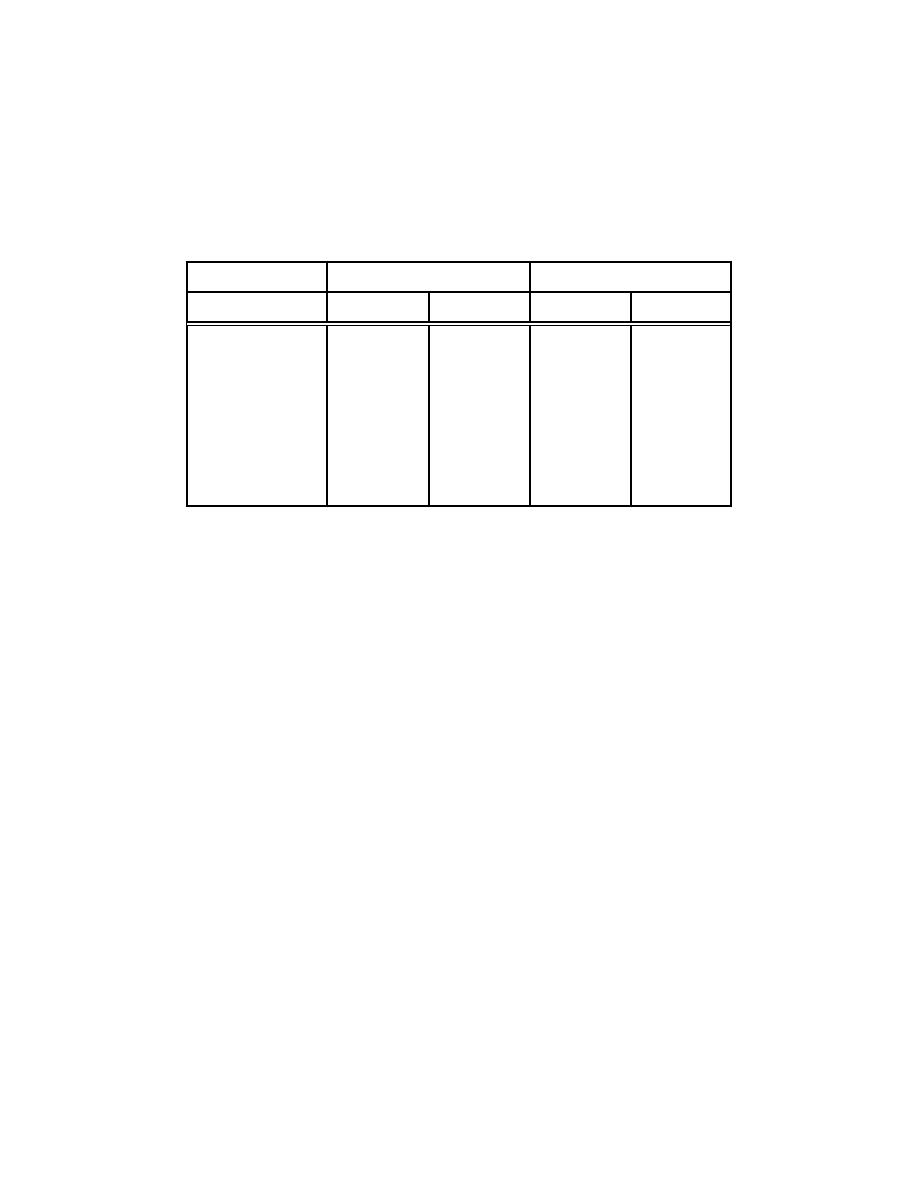
Table 3-12. M easured ARFs From Burning Sm all Volum es of
30% TBP-Kerosine Traced with Selected Radionuclides
(Table I - M ishim a and Schwendim an, June 1973)
ARFs Self-Extinguishm ent
ARFs Com plete Dryness
1-cf m
2-cf m
1-cf m
2-cf m
Uranium
2.7E-4
2.3E-4
-----
3.0E-3
Cesium
2.2E-3
2.5E-3
1.9E-3
1.0E-2
Cerium
7.4E-3
5.6E-3
7.7E-3
7.1E-3
Zirconium
6.5E-3
ND
5.5E-3
2.4E-3
Iodine
6.57E-1
6.53E-1
8.17E-1
8.43E-1
8.28E-1
8.33E-1
as volatiles. Volatiles of interest for phenomenological stresses in nonreactor nuclear
facilities are typically limited to iodine (NUREG-1320, NUREG-1140).
3.3.2
P ool F ires of 30% T B P-K erosin e
The ARFs for strontium from a large-scale 30% TBP-kerosine burn were reported by Sutter,
Mishima and Schwendiman (June 1974). One hundred and fifty liters (150 l) traced with
25 g of strontium nitrate were burned in ten 17-in. x 23-in. x 3-in. deep stainless steel pans
placed on concrete block above an 8-in. pool of water on the floor of a 12-ft x 12-ft cell of
insulating board held in a sheet steel silo. The combustible organic phase was not in contact
with an aqueous phase. Kerosine was floated on the surface of the water pool to aid in the
burning of the 30% TBP-kerosine PUREX-type solvent. The organic liquids were ignited
and the airborne materials carried to the exhaust gas treatment/sampling train apparatus
shown in Figure A.9 in Appendix A taken from the reference document (Figure 1 - Sutter,
Mishima and Schwendiman, June 1974). Two of the three burns generated usable data with
ARFs of 2.2E-3 and 1.9E-3. The values are generally consistent with those generated in the
small volume/surface area experiments in subsection 3.3.1.1.
3.3.3
C om b u stion of T B P-K erosin e S olu tion s O ver P ools of A cid , V igorou s B oiloff
Halverson, Ballinger, and Dennis (February 1987) reported measurements of airborne
uranium during the burning of combustible organic liquid over aqueous solutions. Small
volumes of liquid were placed in metal beakers (except in one case where a borosilicate glass
beaker was used to minimize the heat transfer through the beaker) on a load cell as shown in
Page 3-44
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |