 |
|||
|
|
|||
|
Page Title:
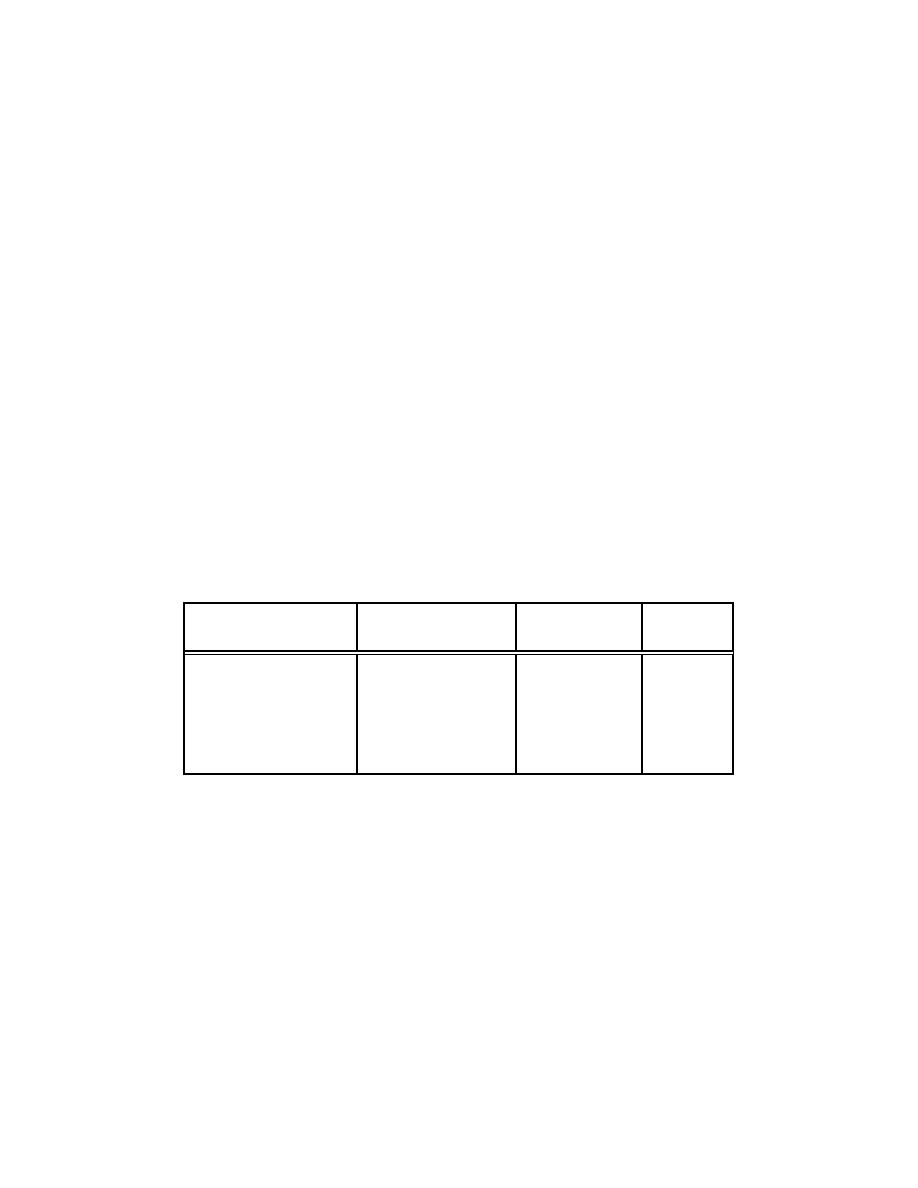
Table 4-4. M easured ARFs During Self-Sustained Oxidation of Unalloyed Plutonium M etal in Flowing Air |
|
||
| ||||||||||
|
|  DOE-HDBK-3010-94
4.0 Solids; Metals
the highest ARF (2E-3 at 950 oC in air) with the RF provided by the authors of 0.001 (in
Figure A.22, Appendix A), the respirable source term (ARF x RF = 2E-6) is less than that
measured by Mishima (November 1966) of ~ 3E-4 (5E-4 x 0.5 = 2.5E-4).
Mishima (December 1965) measured the airborne release during the oxidation of unalloyed
plutonium metal at elevated temperatures in flowing air and the size distribution of the
residue. Right cylinders of unalloyed plutonium metal (0.594 cm to 0.625 cm in diameter by
1.73 cm to 1.89 cm long, weighing 9.89 g to 11.34 g), were heated to temperatures
exceeding the ignition temperature of the metal. Ignition temperatures ranged from 490 oC
to 500 oC with temperatures (measured above the oxidizing specimen by thermocouple)
during the complete oxidation of the specimens ranging from 410 oC to 900 oC. Air, at
predetermined velocities ranging from 3.3 to 50 cm/s, was passed over the oxidizing
specimen and particles entrained from the oxidizing mass were collected on a membrane
filter. The size distribution of the powder residue was determined by a combination of
sieving and air elutriation. The experimental apparatus is shown schematically in Figure
A.15 and the measured results are reproduced in Table A.24 in Appendix A. The pertinent
data are shown in Table 4-4.
Table 4-4. M easured ARFs During Self-Sustained Oxidation of Unalloyed
Plutonium M etal in Flowing Air
(Table III - M ishim a Decem ber 1965)
Sam pling Duration
Tem perature Range
Air Velocity
o
C
ARF
(m in)
cm /s
155
Amb
to
900
C
3.3
2.8E-8
74
Amb
to
560
C
13.5
3.1E-7
75
Amb
to
650
C
50.0
5.3E-7
146
Amb
to
650
C
3.3
4.1E-8
153
Amb
to
560
C
3.3
2.6E-7
117
Amb
to
560
C
20.0
3.1E-8
The values appear to be lower than the ARFs reported by Carter and Stewart (1970) for the
airborne release during oxidation after ignition. The highest ARF is ~ 5E-7, an
order of magnitude less than the geometric mean value specified by Carter and Stewart
(1970). The measurements are limited and do not appear to be strongly influenced by any
measured parameter (temperature, air velocity). A possible factor is the limited convective
flow to entrain any particles ejected from the oxidizing mass due to the limited size of the
specimen and the presence of a boat that may partially shield the oxide mass from the
airflow. If the measurements represent almost comparable conditions, experimental
Page 4-23
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |