 |
|||
|
|
|||
|
Page Title:
Figure 4 Alpha particle (or nucleus of a helium atom) |
|
||
| ||||||||||
|
|  Tritium Primer
DOE-HDBK-1079-94
RADIOLOGICAL FUNDAMENTALS
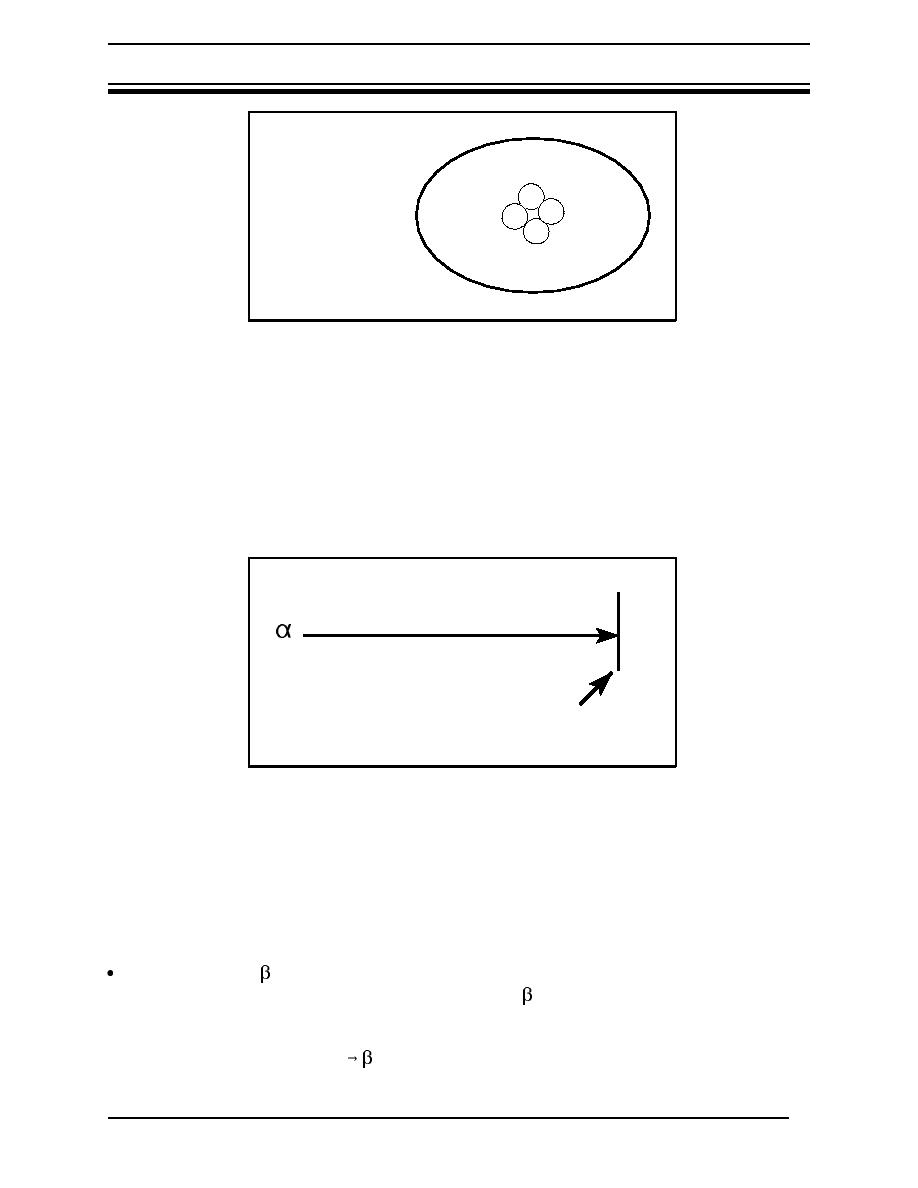
2 neutrons, 2 protons
no electrons
+n
n
+
Figure 4 Alpha particle (or nucleus of a helium atom)
to ionize nearby atoms as it passes through body tissue. The strong positive charge and its
relatively slow speed (resulting from its large mass) causes the alpha particle to interact strongly
with orbiting electrons of atoms and molecules and to lose large amounts of energy in a short
distance. This limits the penetrating ability of the alpha particle, making it easy to stop. A few
centimeters of air, a sheet of paper, or the outer layer of skin stops alpha particles (Figure 5).
Paper or outer layer of skin
Figure 5 Alpha shielding
Alpha particles are not an external radiation hazard because they are easily stopped by protective
clothing or the outer layer of skin. However, if an alpha emitter is inhaled or ingested, it becomes
an internal radiation hazard. Because the source is in close contact with body tissue, the alpha
particle will dissipate its energy in a short distance of the tissue.
beta particle ( )--is equivalent to an electron except for its source. Beta-emitting
nuclides have too many neutrons. A neutron emits a particle, and the neutron is then
converted to a proton. Tritium decay provides a good example of this process:
3
+ 3He + energy .
H
1
2
Rev. 0
Page 7
Tritium
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |