 |
|||
|
|
|||
|
|
|||
| ||||||||||
|
|  Tritium Primer
DOE-HDBK-1079-94
RADIOLOGICAL FUNDAMENTALS
RADIOLOGICAL FUNDAMENTALS
This section provides a review of radiological fundamentals. The reader is assumed to be
familiar with this information from radiological worker training. The section discusses hydrogen
and its isotopes and describes basic radiological concepts.
Hydrogen and Its Isotopes
Atomic nuclei of a particular element (such as hydrogen or oxygen) have the same number of
protons (positively charged), but may have a different number of neutrons (no net charge).
Those that have a different number of neutrons are isotopes of that element. Most elements exist
hydrogen either have no neutrons (normal hydrogen, called protium), one neutron (deuterium),
chemical properties, the nuclear properties can be quite different.
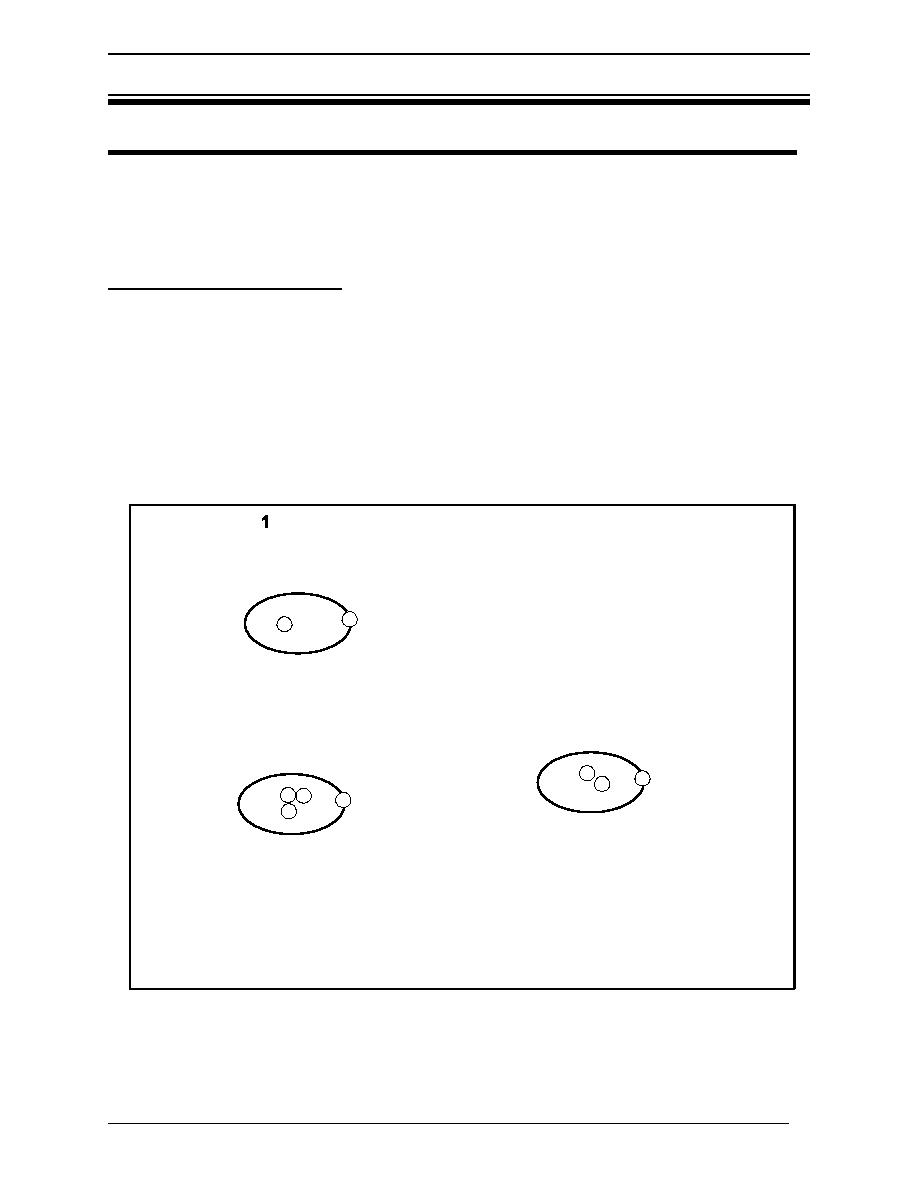
1. Protium - 1H (1 proton, 1 electron)
-Stable (not radioactive)
-Comprises 99.985% of natural hydrogen.
-
+
2
2. Deuterium - 1H or D (1 proton, 1 neutron, 1 electron)
-Stable (not radioactive)
-Comprises 0.015% of natural hydrogen.
+n
-
+n
-
n
3
3. Tritium - 1 H or T (1 proton, 2 neutrons, 1 electron)
-Radioactive -18
-Comprises 10
parts of natural hydrogen.
Figure 1 Hydrogen isotopes
Rev. 0
Page 3
Tritium
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |