 |
|||
|
|
|||
|
|
|||
| ||||||||||
|
|  Tritium Primer
DOE-HDBK-1079-94
RADIOLOGICAL FUNDAMENTALS
release 12 106 Ci/yr, a small fraction of which comes from light-water reactors. Tritium is
also a by-product of light-water and heavy-water nuclear reactor operation. In their coolants,
these reactors produce about 500 to 1,000 and 2 106 Ci/yr, respectively, for every 1,000
MW(e) of power. Tritium is a fission product within nuclear fuel, generated at a rate of 1 2
104 Ci per year/1000 MW(e). U.S. DOE reactors have produced tritium by the neutron
bombardment of lithium (6Li).
Stable and Unstable Nuclides
In the lighter elements, the ratio of neutrons to protons in their stable nuclei is usually 1 (one
neutron for every proton). For the heavier elements, this ratio gradually increases to about 1.5
(1.5 neutrons for every proton). Although one cannot always predict from its ratio whether an
isotope is stable or unstable, the relationship between the number of protons and neutrons is
extremely important.
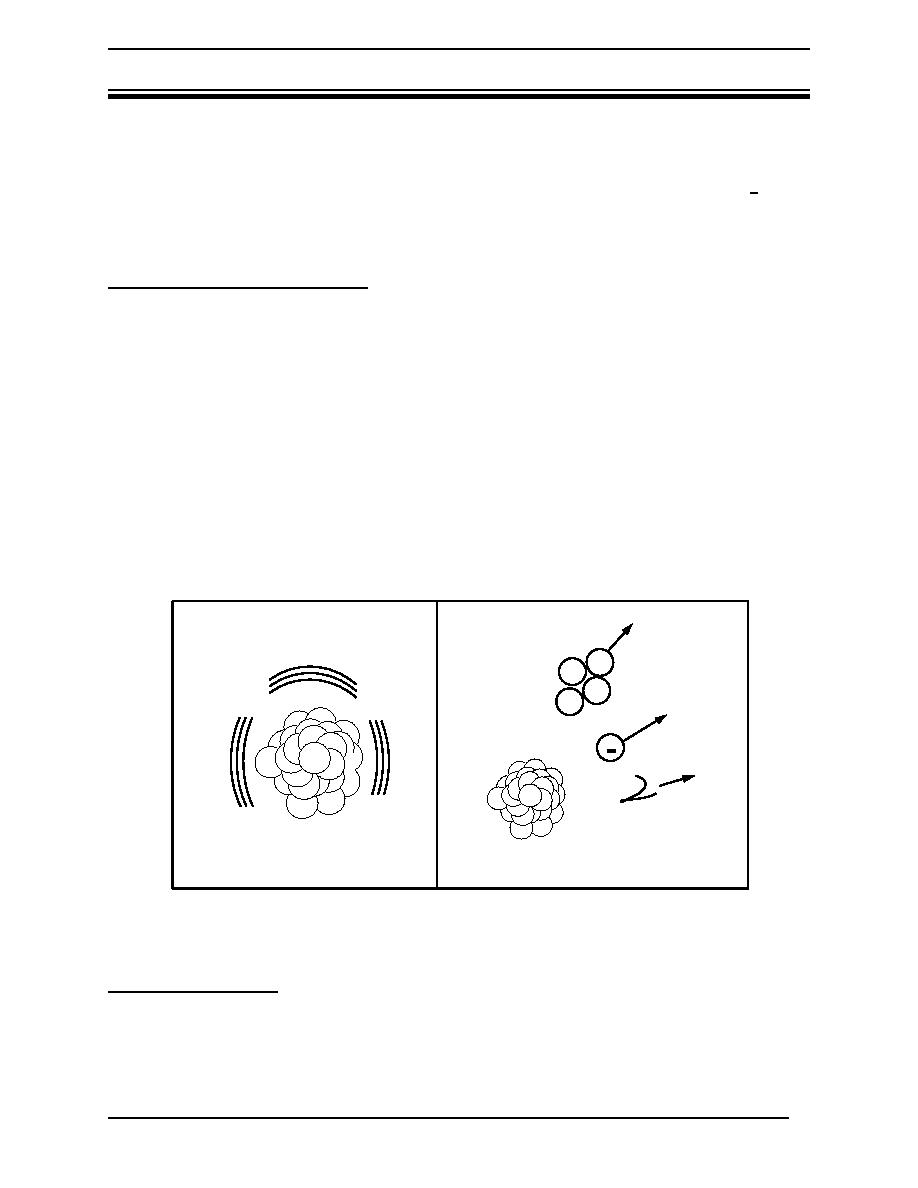
When an isotope is unstable, its nucleus will emit rays or particles or it may split into two
different nuclei. Some combinations of neutrons and protons lead to stable nuclei. If there are
too many or too few neutrons, the resulting nucleus is not stable. This unstable nucleus tries
to become more stable by releasing excess energy. Atoms with unstable nuclei are radioactive.
The process of nuclei releasing this energy is referred to as radioactive decay or disintegration
(Figure 2). If a nucleus is still unstable after radioactive decay, further decay will occur.
Particles emitted
Unstable nucleus
accompanied by
excess energy
n
+
+
n
Figure 2 Radioactive decay
Ions and Ionization
Atoms can combine chemically to form molecules. Atoms and molecules are surrounded by
orbiting electrons (negatively charged). If the number of electrons equals the total number of
protons (positively charged) in the nucleus, the atom or molecule is neutral (uncharged).
Rev. 0
Page 5
Tritium
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |