 |
|||
|
|
|||
|
Page Title:
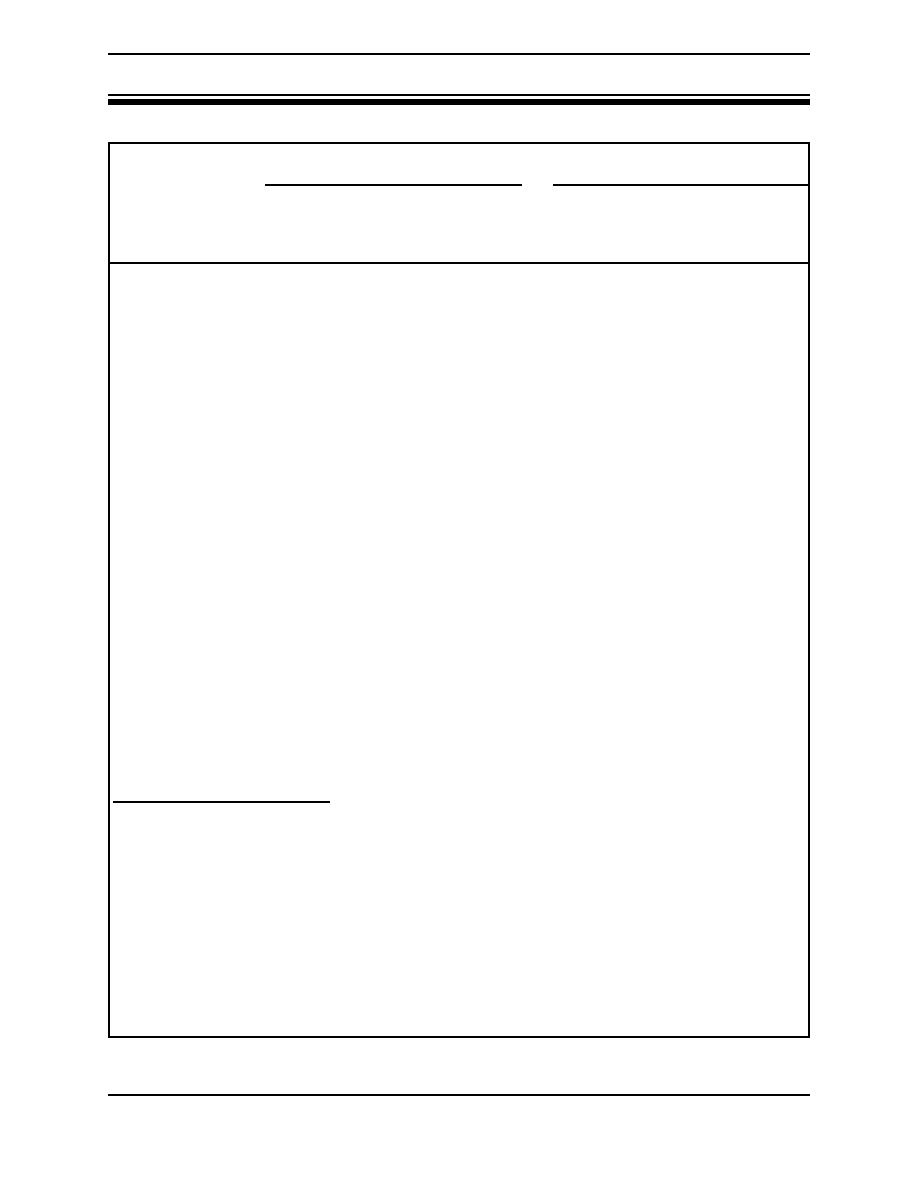
Table 3. Comparison of G-1 and Met-L-X powders. |
|
||
| ||||||||||
|
|  PYROPHORIC METALS
DOE-HDBK-1081-94
Spontaneous Heating and Pyrophoricity
Table 3. Comparison of G-1 and Met-L-X powders.
G-1 powder
Met-L-X
Capable of
Capable of
Capable of
Capable of
complete
control
complete
control
Type of fire
extinguishment
only
Unsatisfactory
extinguishment
only
Unsatisfactory
Dry or oily magnesium
X
--
--
X
--
--
chips or turnings
X1
X2
Magnesium castings and
--
--
--
--
wrought forms
X3
Dry or oily titanium
--
--
X
--
--
turnings
Uranium turnings and
X
--
--
X
--
--
solids
Zirconium chips and
X
--
--
X
--
--
turnings coated with water
soluble oil
Moist zirconium chips and
--
X
--
--
X
--
turnings
Sodium spills or in-depth
X
--
--
X
--
--
X4
Sodium sprayed or spilled
--
--
X
--
--
on vertical surfaces
Potassium or sodium-
X
--
--
X
--
--
potassium alloy spill
X3
X5
Potassium or sodium-
--
--
--
--
potassium alloy fire
in-depth
Lithium spill
X
--
--
X
--
--
X6
Lithium fire in-depth
X
--
--
--
--
Aluminum powder
X
--
--
X
--
--
1. Requires sufficient powder to cover the burning pieces. More agent required than with Met-L-X.
2. Powder clings to vertical surfaces. Unnecessary to bury burning parts.
3. More effective pound for pound than Met-L-X.
4. Adheres to molten sodium on vertical surfaces.
5. Extinguished with difficulty.
6. Powder sinks into molten metal, the sodium chloride reacting with lithium to form lithium chloride and sodium. If continued until
sodium is in excess, the fire can then be extinguished.
Pyrophoricity
Page 42
Rev. 0
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |