 |
|||
|
|
|||
|
Page Title:
Table 2-4. Dissociation pressure equation parameters for palladium hydride and deuteride |
|
||
| ||||||||||
|
|  DOE-HDBK-1129-99
TABLE 2-4. Dissociation pressure equation parameters for palladium hydride and deuteride
Temperature
Range (oC)
Metal
Reference
Investigated
A
B
Tritide
PdHx
Gillespe & Hall
0 to 180
1835.4
7.3278
Gillespe & Hall
200 to 300
1877.82
7.483
Ratchford & Castellan
unspecified
2028.2
7.9776
Wicke & Nernst
-78 to 175
2039
7.65
PdDx
Gillespe & Downs
to 300
1696.11
7.5138
Wicke & Nernst
unspecified
1940
8.00
The 3He generated as a result of decay of the tritium absorbed in the palladium is trapped in the
palladium and is not released until the bed is heated or until the T:3He ratio reaches a particular
value. 3He generated as a result of decay in the overpressure gas is not absorbed in the palladium
and remains in the overpressure gas. Most impurities do not react with, and are not gettered by,
the palladium powder. These impurities accumulate in the overpressure gas as the bed is used to
support operations.
The generation of significant pressure at low temperature (750 psia at 350oC) is the primary
advantage of palladium. The primary disadvantage of palladium is the high partial pressure of
tritium over the powder at room temperature (50 torr at room temperature).
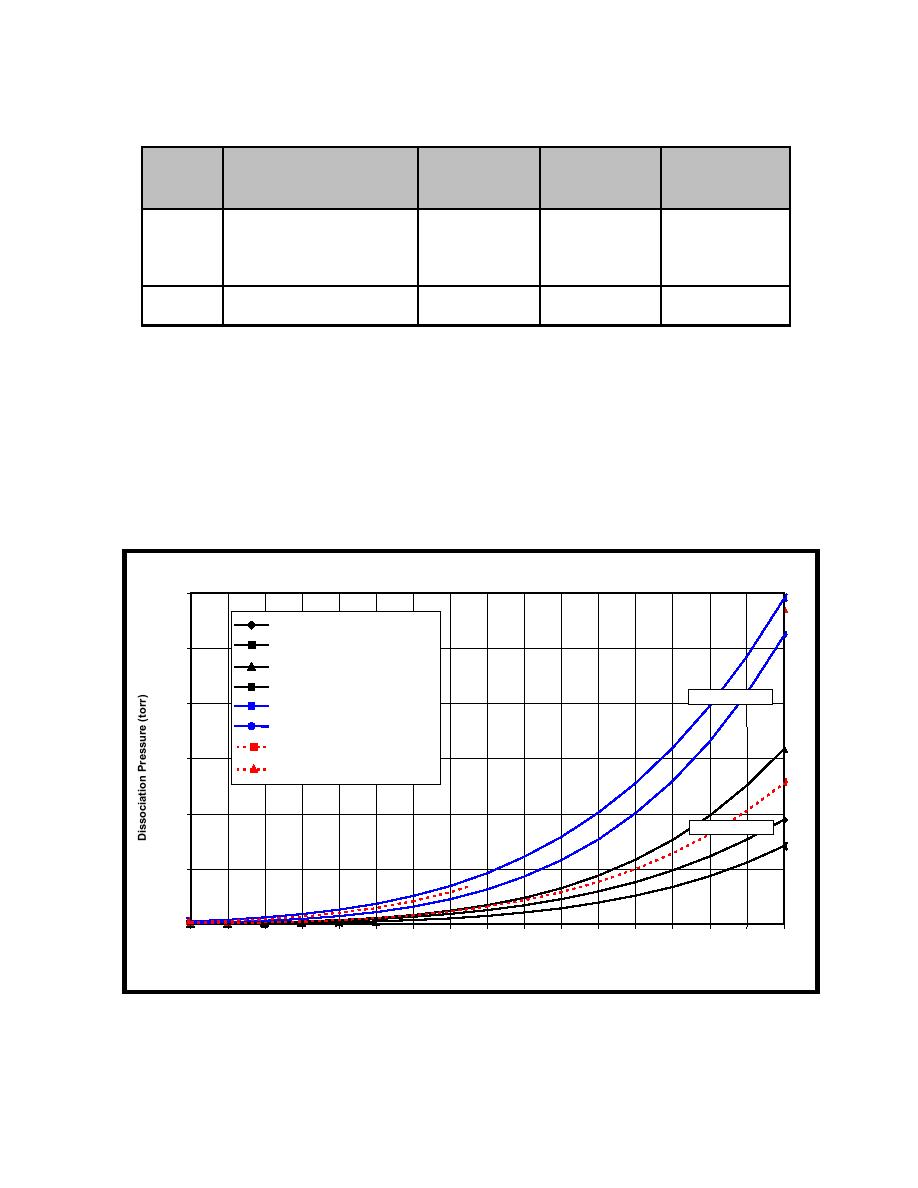
6000
log Pmm=7.3278-1835.4/T
log P m=7.483-1877.82/T
5000
log Pmm=7.9776-2028.2/T
log Pmm=7.65-2039/T
Good Fit Line PdD
4000
log Pmm=7.5138-1696.11/T
log Pmm=8-1940/T
log Pmm=7.6-1900/T
3000
log Pmm=7.75-1810/T
2000
Good Fit Line PdH
1000
0
20
30
40
50
60
100
110
120
130
140
150
160
170
180
70
80
90
Temperature (C)
FIGURE 2-6. Dissociation pressure of palladium hydride and deuteride
13
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |