 |
|||
|
|
|||
|
Page Title:
Tritium Assay Analysis by PVT Mass Spectrometer cont'd |
|
||
| ||||||||||
|
|  DOE-HDBK-1129-99
Rearranging the equation becomes
Moles of Tritium = (n(Moles Total) x m%(Tritium Per Mole Total))/100
The amount of tritium in grams is obtained by multiplying the moles of tritium obtained in the last
equation by the gram molecular weight of tritium (6.0321g).
As an example, the grams of tritium in a shipment of research grade tritium are determined
using the following process.
An analysis of a shipment of research grade gaseous tritium shows the following:
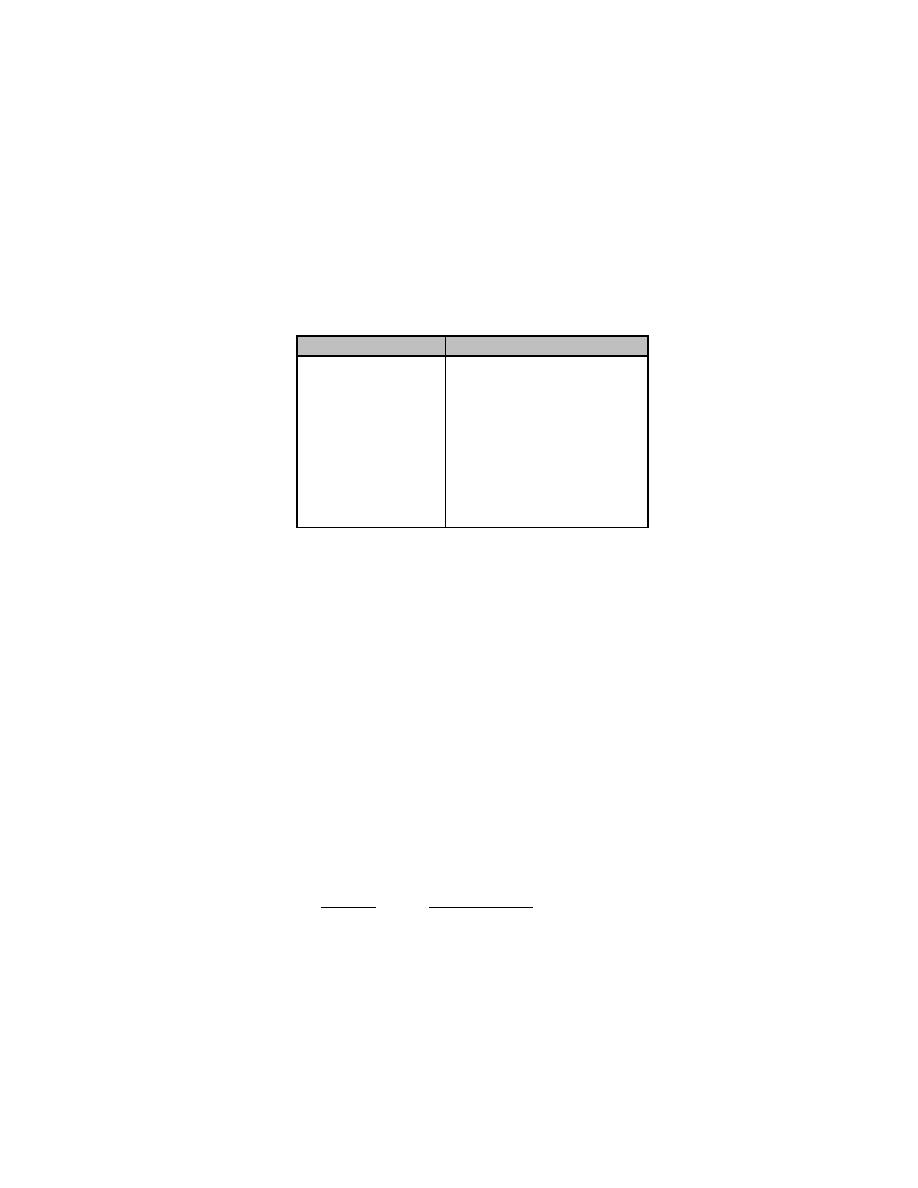
Component
Percent
T2
99.704
0.115
D2
DT
0.079
HT
0.050
HD
0.023
3
He
0.016
Ar
0.012
N2
0.010
H2
0.005
The container pressure is 742 mm, temperature is 20oC, and volume is 49.348 liters. This gives
a compressibility factor (z(T2)) of 1.0006 and the constant, R, is 62.3631.
Calculating the percent tritium is as follows:
m%(Tritium Per Mole Total) = m%(T2) + m%(HT) x 1/2 + m%(DT) x 1/2
= 99.704 + (0.050 x 1/2) + (0.079 x 1/2)
= 99.704 + 0.025 + 0.0395
= 99.7685 percent
The number of moles of gas in the container is calculated by
n(Moles Total) = PV/zRT
= (742 x 49.348)/(1.0006 x 62.3631 x 293.15)
= 2.002 moles total
The amount of tritium in grams is
Grams of Tritium = (n(Moles Total) x m%(Tritium Per Mole Total) x 6.0321)/100
= (2.002 x 6.0321 x 99.7685)/100
= 12.046 grams tritium
The determination of the amount of tritium is only for the point in time that the sample was
analyzed as a result of the decay of tritium. Over time, the number of moles of the gases
containing tritium decrease. The pressure and number of moles of non-tritiated gases increase
with time due to the 3He produced and the molecules formed by the atoms of other materials
released when the tritium decays. Therefore, the mole percent and number of moles of gas in
C-6
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |