 |
|||
|
|
|||
|
Page Title:
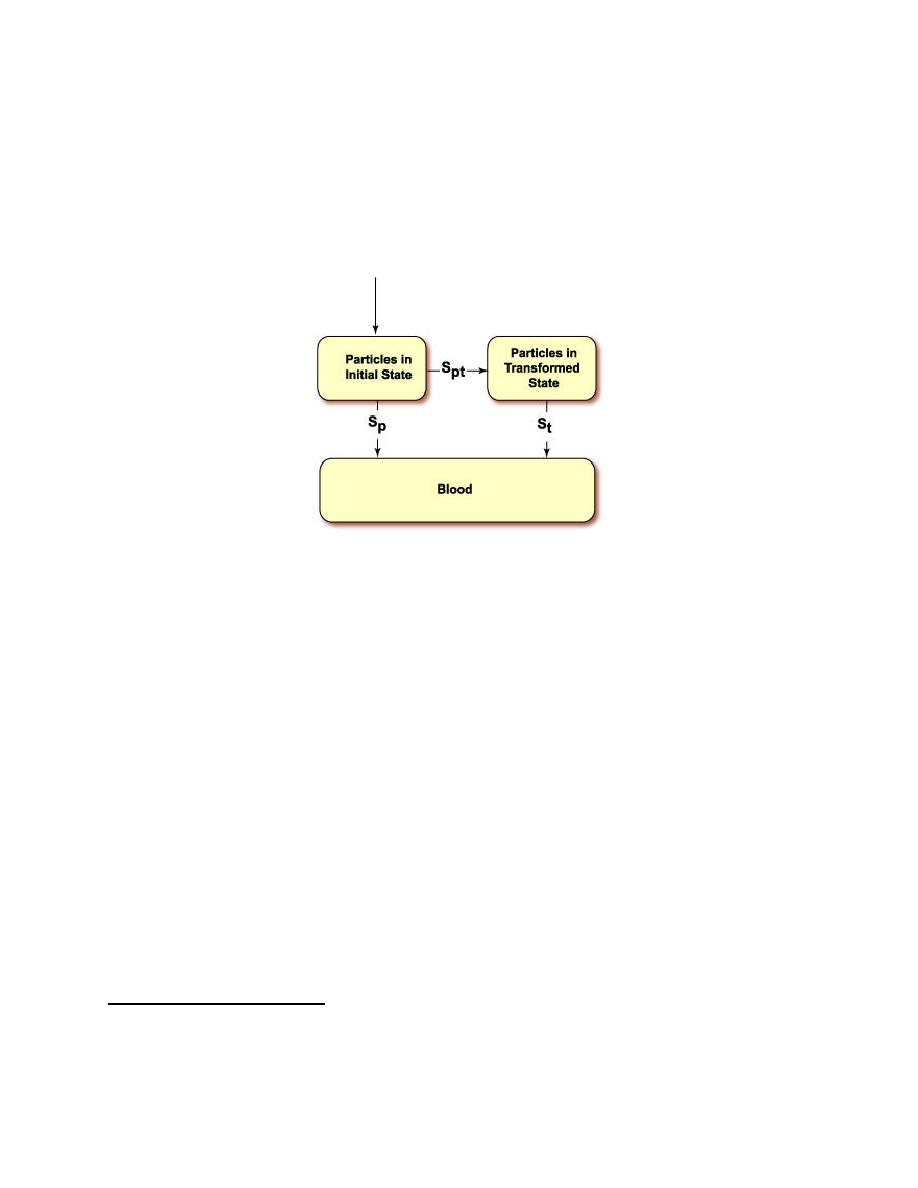
Figure 5-4. Two-stage Dissolution of STC Particles in the Respiratory Tract |
|
||
| ||||||||||
|
|  DOE-HDBK-1184-2004
According to the model, the rate of mechanical clearance is dependent on where
the particle is deposited, but is independent of the chemical form of the particle. On
the other hand, the dissolution rate is dependent on the chemical form of the
particle, but independent of where the particle is in the respiratory tract4. The
dissolution model is shown in Figure 5-4. To account for time-dependent
dissolution a two-stage dissolution model is used. In this model, particles either
dissolve directly (and are absorbed) or are transformed to an intermediate form
before they dissolve.
Figure 5-4. Two-stage Dissolution of STC Particles in the Respiratory Tract.
Default parameters are supplied in ICRP 66 for the mechanical clearance and
dissolution models. The dissolution rate of particles may be classified as Type F
(fast), M (moderate), or S (slow). These classes correlate roughly with the Class
D/W/Y classes in the ICRP 30 respiratory tract model. However, note that D/W/Y
refers to total clearance rates (mechanical plus dissolution) whereas F/M/S refers to
dissolution rates only. The parameters for the time-dependent dissolution model
can be experimentally determined by measuring the dissolution rate in-vitro over
r (t ) = f r ⋅ e - s rt + (1 - f r ) ⋅ e - s st
(Eq. 5-3)
where
r(t) is the fraction of material not dissolved at time t;
fr is the fraction that dissolves rapidly with rate constant sr ; and
ss is the rate constant for the fraction (1 - fr ) that dissolves slowly.
4
Except for the ET1, where no dissolution or absorption is assumed to occur.
5
See ICRP 71, Annexe D, Assignment of Compounds to Absorption Types from Experimental Data.
27
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |