 |
|||
|
|
|||
|
Page Title:
Chemically Nonreactive Compounds |
|
||
| ||||||||||
|
|  DOE-HDBK-3010-94
4.0 Solids; Powders
initial activity collected on the glass fiber filter during the experiment. In some experiments,
portions of the material passing through the chimney were collected on a membrane filter
and sized by optical microscopy using a graticule to determine the size distribution of the
airborne materials. Particles were grouped into seven categories - <5, 5-8, 8-12.5, 12.5-20,
20-32, 32-50 and >50 m equivalent spheres. The size quoted in the text must be
multiplied by the square root of the density of Pu oxide (11.46 g/cm3) to approximate the
AED. These measurements are the basis for the RFs quoted in the tables. In the case of
compounds that were oxidized, the time required to convert all the powder to oxide is not
known. This introduces an additional source of uncertainty into the use of these
measurements for ARFs during the heating and oxidation of the powders. In most cases,
microscopic examination of the residual materials after the heating indicated that for the
conditions under which the higher releases were measured (higher temperatures and air
velocities), the oxidation was relatively complete. Furthermore, the airborne materials were
entrained in an induced flow that probably exceeds that anticipated for convective flow and
bounds the entrainment for convective flow. The pertinent data from these tables for the two
situations are listed in Tables 4-10 and 4-11.
4.4.1.1
Chemically Nonreactive Compounds
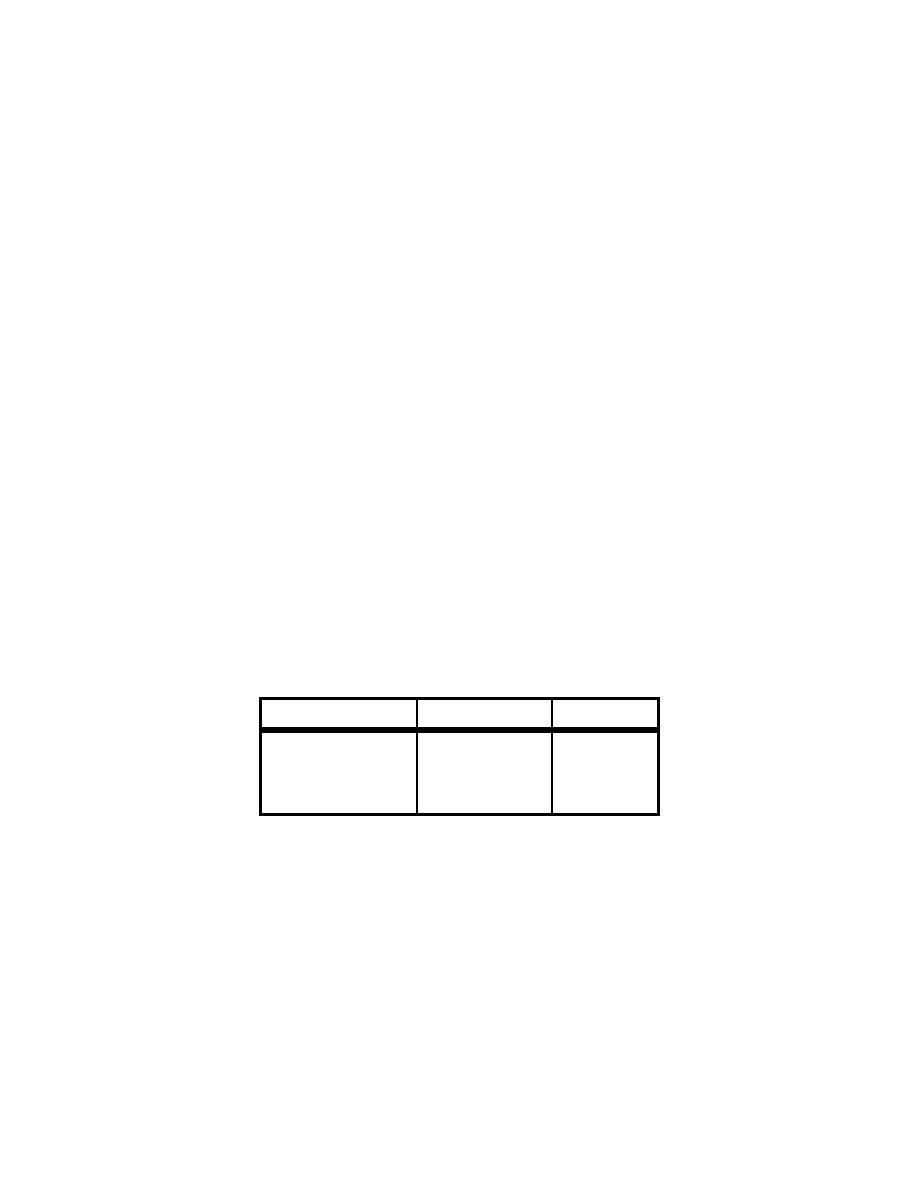
Table 4-10. Airborne Release from Nonreactive Powder During Heating
in Flowing Air
(Table V - Mishima, Schwendiman and Radasch, July 1968)
Temperature, oC
Airflow, m/s
ARF
Ambient
0.1
6.1E-6
1.17
5.6E-3
800-900
0.1
5.3E-6
1.17
2.5E-4
The source material for these experiments was oxide nominally in the size range of 15 to 150
m AED (the upper value is given as 44 m but is the fraction passing through a 325 mesh
screen and is LLD rather than AED for the lower value), although some respirable particles
may have been present. The two values at each separate air velocity appear to be relatively
consistent with the ARF for the higher temperature lower than that at ambient temperature.
Since the lower air velocity (0.1 m/s) is calculated to carry particles as large as 17 m that is
barely above the lower size of the powder used (although it was noted during the oxidation
experiments that the oxide formed was friable), the low ARF value would be anticipated.
Particles as large as 300 m could be carried by the higher velocity (1.17 m/s) and the ARF
Page 4-56
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |