 |
|||
|
|
|||
|
|
|||
| ||||||||||
|
|  DOE-HDBK-1130-98
Module 1: Radiological Fundamentals
Instructor's Notes
c.
Electrons
1)
Electrons are in orbit around the nucleus of an
atom.
2)
Electrons have a negative electrical charge.
3)
This negative charge is equal in magnitude to the
proton's positive charge.
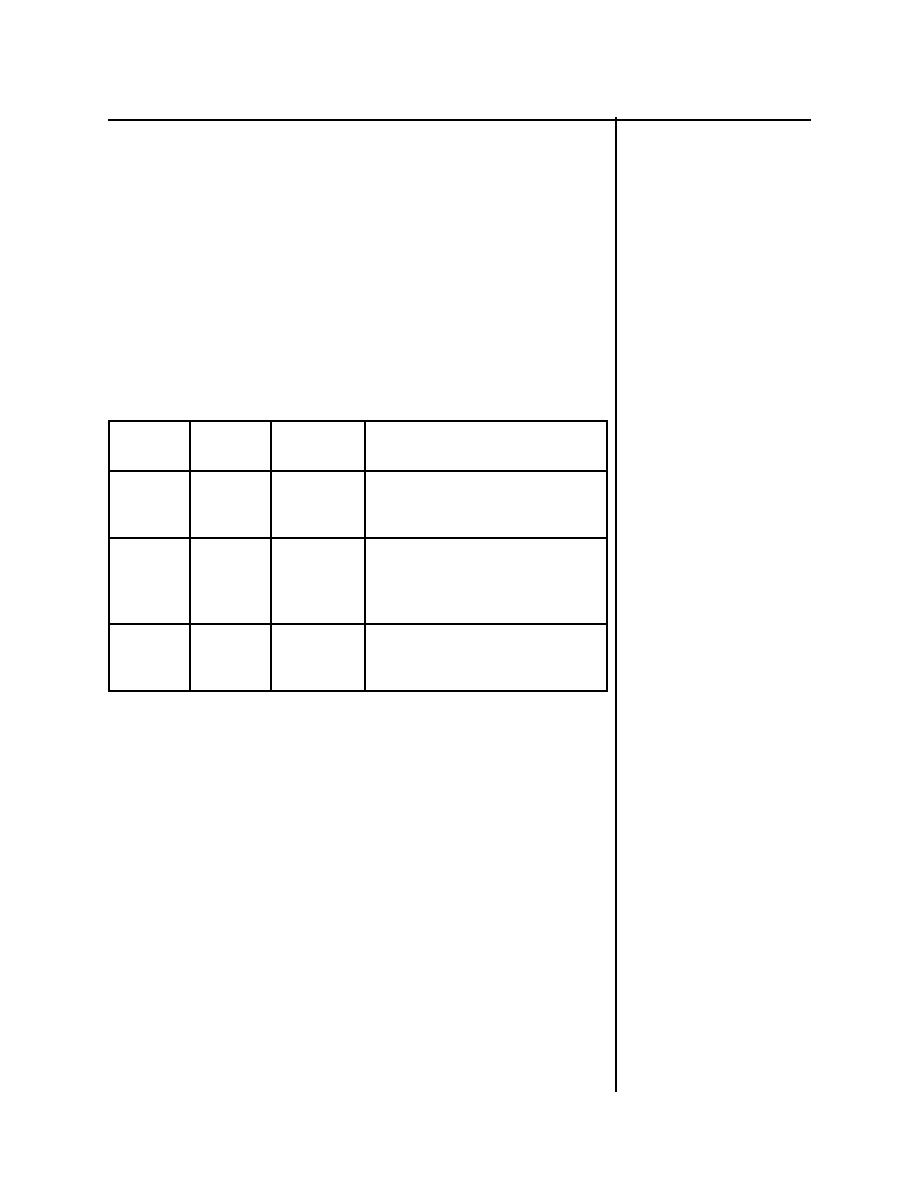
Table 1-1
Basic Particles
3 Basic
Particles
Location
Charge
Comments
Protons
Nucleus
+ (positive)
Number of protons determines the
element. If the number of protons
changes, the element changes.
Neutrons
Nucleus
No Charge
Atoms of the same element have the
same number of protons, but can
have a different number of neutrons.
This is called an isotope.
Electrons
Orbit
- (negative)
This negative charge is equal in
nucleus
magnitude to the proton's positive
charge.
2.
Stable and unstable atoms
Only certain combinations of neutrons and protons result in
stable atoms.
a.
If there are too many or too few neutrons for a given
number of protons, the nucleus will not be stable.
b.
The unstable atom will try to become stable by giving
off excess energy. This energy is in the form of
particles or rays (radiation). These unstable atoms are
known as radioactive atoms.
5
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |